
Savita Nutan, Health and Social Care Lecturer, FSB Croydon
3 million animals in the UK are used in laboratory experiments each year.1 This number does not include the scores of animals bred for research but killed as they are “surplus” and are no longer needed. These experiments performed are either research into basic biology and diseases, assessing the effectiveness of new medicines or safety testing of products ranging from cosmetics to household cleaners for human health and/or environmental safety. Surely these outdated and cruel practices should come to an end?
The animals that are used in various experiments include, but are not limited to, mice, rats, fish, rabbits, guinea pigs, hamsters, birds, cats, dogs, mini-pigs, and non-human primates (monkeys, and in some countries, chimpanzees). All animal laboratory procedures (even those classed as “mild”) can cause physical and emotional distress and suffering. Imagine a sentient animal, with the ability to have feelings, being caged for the rest of their lives and either undergoing painful procedure(s) or seeing these procedures being done to a fellow animal. For that reason alone, we should be looking towards the end of animal testing.

Sadly, if that were reason enough, we would have all left animal testing a long time ago. However, the argument purported by the animal research community is that animal testing is necessary to test these chemicals, including medicines, for the safety of human health.
What if I told you animals hold no guarantee that these chemicals will be safe for us humans? I have studied genetics and worked within the NHS identifying and reporting genetic diagnoses and even a single mutation in our DNA (the genetic code which makes us who we are) can cause such profound symptoms of a disease. So, what makes us believe that other species of animals, who are quite different from us at a DNA level, will prove a product is safe for humans? These differences at the DNA level between us and them are imperative when considering how effective a medicine is and, equally important, which chemicals are toxic to human health.
Around 90%3 of clinical drug development fails, meaning approximately 1 in 10 drug candidates successfully get regulatory approval. With the average costs of these clinical trials ranging between $1–2 billion, the pressure is on to successfully trial a candidate drug. Replacing animals in science is therefore no longer an ethical question. It’s a vital race against time for the betterment of human health. So naturally you ask, how do we replace animals in science with better, more effective techniques?
Scientists, medical professionals, and animal welfare advocates across the globe have been instrumental in answering the big question. Here in the UK, we are lucky to have charities like Animal-Free Research UK, to support scientists aiming to replace animals in medical research and move towards the future with non-animal methods (hereafter shall be referred to as NAMs).
“Replacing animals in science is therefore no longer an ethical question. It’s a vital race against time for the betterment of human health.”
Having personally left a career in medical research and diagnostic genetics behind, my resolve to champion animal-free science is further strengthened upon hearing the outstanding progress in NAMs. And this two-day conference set in the picturesque Madingley Hall, home to the University of Cambridge Institute of Continuing Education (ICE), did not disappoint.
Day One: Innovation in NAMs Research and Technology

We need to deconstruct the big question, if we want to replace animals in science, we need to look at how the science is conducted. Are we replacing a whole animal in the procedure with a different “model” or are we replacing the ingredient or material in the experiment that was derived from an animal? Developing accurate testing “models” which either replaced the animal or animal-derived ingredient and accurately captured the human disease in question was what the first day of the conference was all about. Exciting talks from leading animal-free biotechnology companies and researchers from universities in the UK and abroad had covered innovative replacement models and materials.
Interesting to note that there are several diseases for which the animal model is completely unsuitable. Take mesothelioma, for example, an aggressive form of cancer developing in the lining which covers certain organs in the body4. It is particularly linked to asbestos exposure and more than 2,700 people are diagnosed each year in the UK. The problem with current models of this deadly disease, such as genetically modified mice or asbestos-induced tumours, is that drivers of disease progression cannot be accurately recapitulated.
To address this problem, Dr Fiona Murphy and the team at the University of Strathclyde presented the development of a human organ-like model grown in a dish, completely outside of a human body, known as ‘mesobags’. Mesothelioma cells can grow in this 3D matrix and the application of microfluidic technology enables high control of fluids such as nutrients. This approach enables tumour development to occur organically, accurately highlighting potential pathways which can be the target of clinical drugs. This platform can be scaled up and in the future patient-derived cells could be utilised to truly personalise medicine.
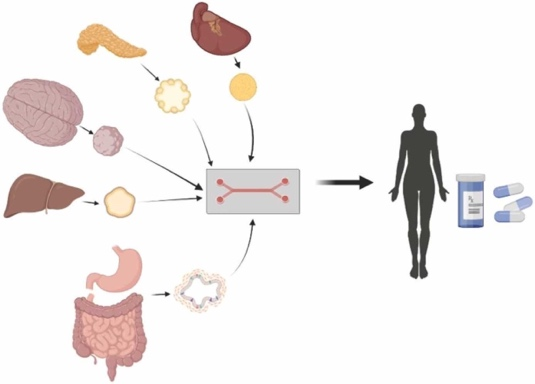
Heart, pancreas, brain, liver and gastrointestinal tract “organoids-on-a-chip” are powerful tools for modelling the human body and useful for drug testing.5
Recreating human tissues and organs into 3D models is a powerful tool and, in addition to drug discovery, it has a range of applications such as screening drugs for potential side effects. For example, drug-induced liver injury (DILI) is the leading cause of liver toxicity and is the leading cause of failure in the drug development process7.
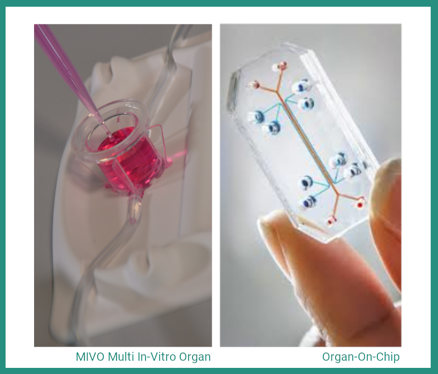
We are now in the exciting era of multi-organ modelling, where we can create synthetic models of multiple organ-like tissues connected to each other, with organ-on-chip technology. With applications ranging from modelling multi-organ system diseases to the spread of cancers, Maurizio Aiella, CEO of React4Life, and their product MIVOTM (image left)10 shows just how far we can go without the need for animals.
What surprised me about the list of companies being represented at the conference, was the inclusion of Unilever. This parent company owns several well-known brands, from Dove to Ben & Jerry’s ice cream. What we are seeing with these big corporations, Procter and Gamble (P&G) included11, is that whilst many brands they own are certified cruelty-free, they are certainly not a cruelty-free parent company. This is highlighted by their own position statement on alternative approaches to animal testing:
“Occasionally, across Unilever’s broader portfolio of brands, some ingredients that we use must still be tested by suppliers by law, to comply with regulatory requirements in some markets; and some government authorities test certain products on animals as part of their regulations.”
A country may require animal testing by law, such is the case in mainland China, but there is no requirement for the company to sell there. Hence, can we really say that these corporations such as Unilever and Procter and Gamble are pushing forward the animal-free revolution? Or are they supporting animal-free safety assessment where necessary by law and presenting themselves as an animal-free championing company?
Day 2: Changing the Paradigm
Day 2 opened with a fantastic keynote talk from the Pioneer Award winner, Dr Merel Ritskes-Hoitinga. One of the key takeaways was how the COVID-19 vaccine timeline was fast-tracked by reducing the number of animal studies and promoting alternative methods. Public health disasters have long held the key to forcing acute decisive regulatory action. With hope for the future, Dr Merel highlighted the transition at the government level was already underway with the US FDA Modernisation Act 2.0 allowing animal replacement testing to be accepted in drug development pathways. After having worked on a multitude of projects to secure an animal-free future, she encourages us all with her words. “Change coincides with resistance. It needs perseverance and managing transitions”. We now must place pressure on the EU Parliament for a roadmap out of animal testing.
“Change coincides with resistance. It needs perseverance and managing transitions”.
Throughout the conference I wondered, with its clearly enormous potential, why aren’t NAMs present in mainstream academia? After all, we were discussing this in a University of Cambridge venue, a university with a global reputation for outstanding academic achievement. 8 Among the answers received to this question one of them was that staff expertise is sorely needed to set up these complex and challenging methods. Here at the De la Roche lab, department of Biochemistry at the University of Cambridge, a pioneering non-animal method was introduced. An unprecedented organoid (organ-like) technique was developed which is completely free from animal ingredients. In the poster presented at the conference Professor Marc de la Roche and team have been looking into how colorectal cancer can be modelled using this novel animal-free organoid effectively, with implications for future cancer research.
On the one hand, there’s an unprecedented NAM poised to outperform animal studies, and on the other is the academic institution, the University of Cambridge. In 2022 the university of Cambridge was the 2nd organisation in Great Britain to carry out the largest number of procedures on living animals for medical, veterinary, or scientific research. 9 And while the institution continues to defend animal research, real progress is stunted. Staff expertise is limited to a very small number of individuals, and it’s the university that ultimately loses.
The future is animal-free. Not just for the safety of animals but, for the safety of humans, we need better solutions. Conferences like these are imperative to bring together creative thinkers for the centuries-old problem of animal testing.
References
- Humane Society International. About Animal Testing [Internet]. Humane Society International. 2012. Available from: https://www.hsi.org/news-resources/about/
- Shegokar R. Preclinical testing—Understanding the Basics First. In: Shegokar R, editor. Drug Delivery Aspects. 2020. p. 19–32.
- May DL 9, 2019. The Latest on Drug Failure and Approval Rates [Internet]. In the Pipeline. 2019. Available from: https://blogs.sciencemag.org/pipeline/archives/2019/05/09/the-latest-on-drug-failure-and-approval-rates
- NHS. Mesothelioma [Internet]. nhs.uk. 2017. Available from: https://www.nhs.uk/conditions/mesothelioma
- Saorin G, Caligiuri I, Rizzolio F. Microfluidic organoids-on-a-chip: The future of human models. Seminars in Cell & Developmental Biology. 2022 Oct;144.
- Fröhlich E. Replacement Strategies for Animal Studies in Inhalation Testing. Sci [Internet]. 2021 Dec 1 [cited 2022 Mar 29];3(4):45. Available from: https://www.mdpi.com/2413-4155/3/4/45
- CN Bio. Ready to Learn? Organ-on-a-Chip Resources – CN Bio [Internet]. cn-bio.com. 2020 [cited 2023 Oct 25]. Available from: https://cn-bio.com/resources/
- University of Cambridge. University of Cambridge [Internet]. University of Cambridge. 2019. Available from: https://www.cam.ac.uk/
- University of Cambridge. UK organisations release annual statistics for use of animals in research [Internet]. University of Cambridge. 2023. Available from: https://www.cam.ac.uk/research/news/uk-organisations-release-annual-statistics-for-use-of-animals-in-research-0
- React4Life. MIVO® and microfluidic chip in pharmaceutical analysis [Internet]. React4Life. 2021 [cited 2023 Oct 25]. Available from: https://www.react4life.com/mivo-and-microfluidic-chip-in-pharmaceutical-analysis/
- Procter and Gamble. Procter & Gamble Company [Internet]. www.pg.co.uk. Available from: http://www.pg.co.uk
